Using CoGe for the analysis of Plasmodium spp
About this guide
This 'cookbook' style document is meant to provide an introduction to many of our tools and services, and is structured around a case study of investigating genome evolution of the malaria-causing Plasmodium spp. The small size and unique features of this pathogen's genome make it ideal for beginning to understand how our tools can be used to conduct comparative genomic analyses and uncover meaningful discoveries.
Through a number of example analyses, this guide will teach users about the following tools:
- LoadGenome: Add a new genome to CoGe.
- LoadAnnotation: Add structural and/or functional annotations to a genome.
- GenomeInfo: Get information about a genome.
- GenomeList: Get information about several genomes in a table.
- CoGeBLAST: BLAST against any set of genomes.
- GEvo: Microsynteny analysis.
- SynMap: Whole genome syntenic analysis.
- - #Calculating and displaying synonymous/non-synonymous (Ks, Kn) data: Characterize the evolution of populations of genes.
- - SPA tool: Syntenic Path Assembly to assist in genome analysis.
- SynFind: Identify syntenic genes across multiple genomes.
- CodeOn: Characterize patterns of codon and amino acid evolution in coding sequence.
A brief introduction to Plasmodium genome evolution
The study of parasitic genomes via comparative genomics offers many unique challenges. Parasite genomes are characterized by a combination of gene loss and the acquisition of species- or lineage-specific genes; in particular, many specialized genes mediate host–parasite interaction [1]. The dynamic nature of parasitic genomes is particularly evident within the genus Plasmodium. The genus emerged ~40 million years ago and harbors roughly 200 species of parasitic protozoa better known as malaria parasites. All Plasmodium species have a complex life cycle involving some kind of vertebrate host and a mosquito vector of the genus Anopheles (mammals) or Culex (birds). In addition, Plasmodium species share similar life cycle characteristics, albeit with a few exceptions (e.g. hypnozoites). However, host and vector preferences differ among Plasmodium species [2].
Plasmodium genomes are tiny (between 17-28Mb) in comparison to those of their vertebrate (1Gb for birds; 2-3Gb for mammals) and mosquito (230–284Mbp) hosts [3]. All Plasmodium genomes consist of fourteen chromosomes (nuclear genome), as well as a mitochondrial and apicoplast genome. Despite these shared genomic characteristics, the structural organization, gene content, and sequence of Plasmodium genomes is highly variably within the genus [4]. The exact origins and mechanisms of these differences remain largely unexplored, however, they are generally hypothesized to stem from host shift events [5][6].
An increase in funding devoted to malaria research has coincided with a dramatic increase in publicly available genomic information for Plasmodium [7]. The most prominent repository is found in NCBI/Genbank [8]; while additional and unique sequences can also be found on other databases: PlasmoDB [9], GeneDB [10], and MalAvi [11]. This wealth of genomic data facilitates detailed comparative genomic approaches, opening the possibility to:
- Infer origins of certain traits, specialized phenotypes, and genomic features.
- Track the maintenance of conserved genes across the genus, as well as the gain or loss of genes unique to a single species or a group of closely related species.
- Identify the potential historical interactions that might have lead to the development of genomic adaptations.
One of the many remarkable trends of Plasmodium genome evolution is the rapid change in GC content. Plasmodium falciparum and closely related parasites have a remarkably AT-rich genome compared to other Plasmodium species [12]. While significant shifts in GC content have been reported in other parts of the tree of life such as Bacteria [13][14] and monocots [15], the short evolutionary time during which this change has occurred in Plasmodium is noteworthy. Moreover, the GC content variability observed amongst Plasmodium species has not yet been observed within other closely related genera. AT-rich genomes are not only challenging to sequence relative to GC-rich genomes[16], but also differ in codon usage, patterns of genome mutability, and evolution of repetitive elements. A comparative genomic approach makes it possible to assess the evolutionary origins and trace the patterns of GC content shift across the Plasmodium genus.
Perhaps one of the most significant aspects of Plasmodium evolution, and of parasites in general [17], are the evolution of multigene families. Within Plasmodium numerous multigene families show specific patterns of gene gain/loss. The differences in the ancestry of these families are also noteworthy, with many gain/loss events being observed either in a single species [18], a single clade, or the entire Plasmodium genus. In this sense, each multigene family illustrates a different aspect of the evolutionary history of Plasmodium and its adaptation to different hosts and vectors.
Through a case study on Plasmodium evolution, we will illustrate how CoGe can be used for the analysis of multigene families, local synteny, and whole genome comparisons (genome composition, rearrangement events, and gene order conservation).
Finding and integrating genomes in CoGe
An increasing number of Plasmodium genomes have been sequenced in recent years, a number that will likely increase in the future. Thus, tools that permit rapid integration of genomic information and its subsequent analysis are essential for Plasmodium research. Online platforms aid in reducing computational time, costs, and foment worldwide collaborations. CoGe is one of these platforms.
The first step in analyzing Plasmodium genomes with CoGe is determining which genomes are already included in the data repository.
Finding the Plasmodium genomes already present in CoGe

A significant accomplishment in the study of Plasmodium genomics was the full sequencing and assembly of the P. falciparum genome [19]. Over the years, this genome has been revised and re-annotated, resulting in different "releases", or versions of the P. falciparum genome. CoGe’s repositories contain each of these releases with a unique version identifier (i.e., v5, v4, etc). This happens because the CoGe platform incorporates new versions of a genome without deleting previous ones. Thus, you can find the initial P. falciparum sequenced genome loaded onto CoGe (v3) alongside the more current releases (v5).
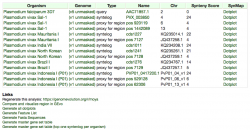
Before importing a genome into CoGe, and to prevent redundancy of genomic information, it is recommended to identify what data has previously been imported. You can search CoGe’s Plasmodium genomes by typing the word "Plasmodium" into the Search bar at the top of most pages (Figure 1). This will retrieve all organisms and genomes with names matching the search term. For instance, when searching the term "plasmodium falciparum 3D7", you will see that there are currently eight publicly available genomes associated with this specific strain of P. falciparum. Clicking on any organism will produce the details of the upload. Alternatively, you can find the Tools section on the main CoGe page (Figure 2) and click on OrganismView (https://genomevolution.org/coge/OrganismView.pl).
All publicly available genomes imported into CoGe, and their corresponding metadata, can be found in OrganismView. To search for any genome on OrganismView, type a scientific name into the Search box. The following information will be displayed (Figure 3):
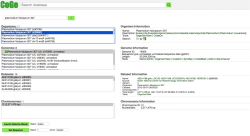
- Organisms: In the case of Plasmodium spp., the different parasitic strains are already imported. In addition, organellar genomes (mitochondrial and apicoplast) have also been imported.
- Organism Information: An outline of the organism's taxonomy (as published on NCBI/Genbank). This section also includes links to some of CoGe's main analysis tools.
- Genomes: All genome versions available. Note that by selecting different genome versions, all associated genomic information changes.
- Genome information: Includes genome IDs, type of sequences uploaded, and sequence length. You can also access CoGe's genome analysis tools in this section.
- Datasets: This section includes the number of datasets for the specified genome. In the case of completely sequenced genomes imported from NCBI/GenBank, it will indicate the chromosome’s accession numbers.
- Dataset information: Provides information for each dataset including accession numbers (if available), the source of the import, chromosome length, and GC%.
- Chromosomes: Shows the number of chromosome in the selected genome. However, depending on the method used to import the genome into CoGe and the dataset itself, the number and length of the chromosomes will vary.
- Chromosome information: Shows each chromosome's ID and lenght on base pairs (bp).
You can find a more detailed description of any genome by accessing the Genome Info section within Genome Information. You can also access links to the majority of CoGe’s comparative analysis tools in this section. Keep in mind that genomes imported to CoGe can be made “Public” or “Restricted”. Genomes made “Public” can be seen and analyzed by anyone using the CoGe platform. “Restricted” genomes can only be seen and/or analyzed by the user and shared accounts (Sharing_data).
Importing Plasmodium genomes into CoGe
If a genome is not found on CoGe's repository then it must be imported before analysis. Genomic data can be imported into CoGe using a variety of methods. We will focus on the two methods most likely to be used when importing genomes. For additional information about other methods please see How_to_load_genomes_into_CoGe. Depending on your intended analyses, you might want to use a complete Plasmodium genome, a specific chromosome, or focus on an organelle. The methods described here can be used to upload either of these data. To import a genome onto CoGe follow these steps:
- 1. Go to the genome database on NCBI/GenBank (or your favorite database) and type "Plasmodium" in the search box.
- 2. In the Representative Genome section you will find links to Download Sequences in FASTA format and Download Genome Annotation (Figure 4).
- - To download a complete Plasmodium genome click on Genome under Download Sequences in FASTA.
- - To download a complete annotation for a Plasmodium genome click on GFF under Download Genome Annotation.
- You can also download single chromosomes and, if available, organellar genomes by clicking on their respective RefSeq or INSDC numbers.
- 3. Go to CoGe and log in. You can follow this link: https://genomevolution.org/coge/
- 4. Click on MyData to reach the Data section of your personal CoGe page (Figure 5). This section will fill up as you import genomes and load Experiments into CoGe.
- 5. Click on NEW and select New Genome from the dropdown menu.
- 6. Input information about the organism's taxonomy and the genome's source on the Create a New Genome window (Figure 6). Consider that taxonomic information for that genome might not have been incorporated into CoGe yet. If this is the case, follow these steps to create a "new organism":
- a. Click on NEW on the "Organism:" section.
- b. Type the scientific name of the organism to be imported on the Search NCBI box. If the organism does not show up select its closest taxonomic relative. In the case of Plasmodium, several strains might be available for a given species (particularly P. vivax and P. falciparum). Make sure to select the correct strain or, if a new strain is being imported, to add its name.
- c. Click Create.
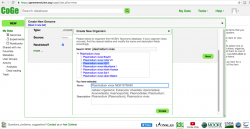
- 7. After creating a new strain/genome, you must also include the import’s metadata. Type the import's genome version in Version after confirming which genome versions are available on CoGe. If this if the first genome imported, the version number should be “1”. Select the sequence type from the dropdown menu on the Type section. Most sequences can be identified as unmasked (check this wiki’s Masked section for further details). Select the Source in the next dropdown menu (in this case NCBI). Finally, tick the check box if you desire your genome to be Restricted.
- 8. Click Next.
- 9. Genome files can be imported to CoGe using four different strategies: 1) import directly from the CyVerse Data Store; 2) create a direct HTP/FTTP link to the data; 3) import the files from a private computer using Upload; and 4) use GenBank accession numbers.
- To import genomes using Upload:
- a. Select a genome file from your local computer and wait for it to be read by CoGe. Once the process is completed select Next.
- b. Click Start to begin the import.
- c. When the import has concluded, the file’s metadata will be visible in the Genome Information page.
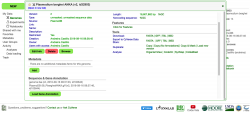
- d. To import annotation data click on Load Sequence Annotation under the Sequence & Gene Annotation menu. Note that any upload can be updated at any point. Thus, genome annotations or experimental data can be added later to any genome already in CoGe.
- e. In the Describe your annotation page, select the version and source of the annotation data and click Next. The data can be uploaded from the CyVerse Data Store, by creating a HTTP/FTP link, or by using the Upload option. Once concluded, the genome annotation should be visible on the Genome Information page under the Sequence & Gene Annotation menu (Figure 7). For more details about uploading genome annotations please check LoadAnnotation.
- To import genomes using NCBI/Genebank:
- a. Select the GenBank accession numbers option. Type or Copy/Paste the RefSeq or INSDC numbers for each chromosome or organelle and click Get. Information from each imported genome should appear under Selected file(s). Once all genomes have been imported (e.g. the 14 Plasmodium chromosomes) click on Next.
- b. Once the import has concluded, the file’s metadata will be visible in the Genome Information page. Note that NCBI/GenBank genome annotations will be automatically imported to CoGe when using this method and that genomes uploaded using this method will be automatically made “Public”.
Exporting genomes from CoGe to CyVerse
- Data can be exported into CyVerse for easy sharing and storage after it has been imported onto CoGe. While this is not required to use any of CoGe's tools, it is a recommended step. You can export data from CoGe into the CyVerse Data Store by following these steps:
- 1. While logged into CoGe, go to the genome's Genome Information page.
- 2. Under the Tools menu, find the Export to CyVerse Data Store option. Click either on the FASTA or the GFF file options to upload genomic data and/or its annotation.
- 3. Wait until the export is completed. From this point forward, your FASTA and GFF files will also be found in the CyVerse Data Store.
Using CoGe tools to perform comparative analyses
Analyzing GC content and other genomic properties (GenomeList)
There are significant variations in average GC content and GC content distribution between the two main human malaria agents: P. vivax and P. falciparum. The average GC content is P. vivax compared to 19.4% in P. falciparum. GC-poor regions are restricted to the subtelomeric regions of P.vivax’s genome, whereas they are ubiquitous across the P. falciparum genome [20]. The current model is that AT-rich genomes represent the ancestral state and GC-rich genomes the derived state in specific Plasmodium lineages [21]. Here, we will evaluate the patterns of GC content variation across three of the four main Plasmodium clades.

CoGe can display a genome’s GC content in GenomeInfo. To calculate GC content, click on %GC under the Length and/or Noncoding sequence sections on the Statistics tab. You can compare and contrast GC content (and other genomic features) across several species and/or strains using GenomeList. This tool creates a list of genomes selected by the user and calculates features such as:
- Amino acid usage.
- Codon usage.
- Coding sequence (CDS) GC content.
- Number of genes.
- Number of introns.
GenomeList also summarizes some of the genomes’s metadata including:
- Sequence type.
- Sequence origin.
- Taxonomy.
- Provenance.
- Genome version.

| The following steps indicate how to perform comparative analyses using the GenomeList tool in CoGe:
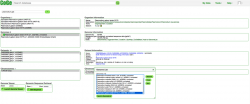
2. Click on OrganismView or follow this link: https://genomevolution.org/coge/OrganismView.pl 3. Type the scientific name of any organism of interest on the Search box. Then, select a genome version. 4. Find the Tools section under Genome Information and click on Add to GenomeList. The first genome added to GenomeList will appear in a new window. 5. Without closing this window, type the scientific name of another organism on the Search box. Select the genome version and click on Add to GenomeList. 6. Once you have added all genomes click on Send to GenomeList (Figure 8). 7. GenomeList will generate a table including all the selected genomes. You can use GenomeList to select and compare different genomic features and attributes. The analyses can be run on specific genomes or on all the included genomes. You can also select the display columns by clicking on Change Viewable Columns. 8. Click on "Send Selected Genomes to" to download the genomes included on GenomeList.
|
Comparing genomic composition sequence: GenomeList
We used GenomeList to compare 12 fully sequenced Plasmodium genomes (Figure 8). Our results show that species closely related to P. falciparum (subgenus Laverania) have similarly AT rich genomes. GC content was higher in Plasmodium species of the simian and rodent clades (Figure 9 and Figure 10). The highest GC content values were observed in species of the simian clade (P. vivax, P. cynomolgi and P. knowlesi). Tellingly, these species all share a common ancestor and diverged from one another recently. GC content varied widely across Plasmodium species infecting humans (P. vivax, P. ovale, P. malariae, and P. falciparum) but not on species infecting rodents (P. berghei, P. chabaudi, and P. yoelii). GC content also varied in human-infecting Plasmodium within the simian clade (P. vivax = 46.89%, P. ovale = 32.83%, and P. malariae = 25.12%). Our results suggest that GC-richness (> 30%) evolved recently and is a derived state within the genus. Our results suggest that a correlation between GC-content and evolutionary relatedness, but not with host-related selective pressures.
AT-richness as an ancestral state for the Plasmodium genus is unusual since closely related genera within the phylum Apicomplexas frequently have GC-rich genomes (Toxoplasma gondii = 52.28%, Cryptosporidium parvum = 30.4%, C. muris = 28.5%, Theileria orientalis = 41.58%, T. equii = 39.47%, Babesia bovis = 36.3%, Eimeria tenella = 51.07%, etc.). Our data suggests that Plasmodium GC content may be in the process of being reinstated to values that can be considered typical for the phylum. The implications of and mechanisms behind the extreme variability in GC-content within Plasmodium are currently being investigated [23].
Identifying gene homologs (CoGeBLAST)
The identification of homology based on sequence similarity is a key tool for gaining insight into an organism’s biology and genetics. Defining evolutionary relationships and inferring common ancestry is particularly challenging when dealing with multigene families. Plasmodium multigene families perform a wide array of functions, have diverse gene organization, and distinct evolutionary histories. Here we focus on a set of multi-gene families arising from the subtelomere (e.g. var, stevor, rifin, or vir) that have very complex evolutionary patterns and organizations [24]. These four gene families are of particular interest because of their role in immune evasion and cell invasion. In addition, these families have undergone rapid sequence evolution and gene turnover [25][26][27]. These factors make inferring orthology/paralogy and gene gain/loss events in Plasmodium subtelomeric families a complex task.
The 313 members of P. vivax’s vir family are grouped into 10 subfamilies based on their sequence similarity. Gene size and structure (number of exons) is largely variable among family members [28][29][30]. The genetic diversity in the vir family is larger than that of other P. vivax families. Only fifteen of the 313 vir genes are shared across all sequenced P. vivax strains despite the recent emergence of the species ~ five million years ago. Within this group, PVX_113230 has been proposed as a potential family founder based on its high sequence conservation [31].
Here we use CoGeBLAST to identify the proposed founder of the Plasmodium vir family (PVX_113230) in six P. vivax strains (including the recently sequenced PO1 strain). CoGeBLAST incorporates genome visualization into BLAST analyses. Therefore, this tool facilitates the study of complex evolutionary patterns.

| The following steps show how to use CoGeBLAST in the CoGe platform:
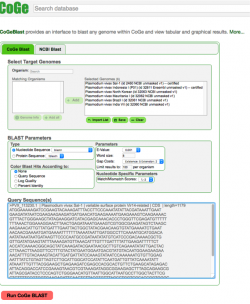
2. Click on CoGeBLAST or follow this link: https://genomevolution.org/coge/CoGeBlast.pl 3. Type the scientific name of the Organism of interest in the Search box. All genomes with names matching the search term will appear under the Matching Organisms menu. Notebooks matching the term will appear in a new window after clicking on Import List. 4. Select all the genomes of interest and click on + Add. The genomes will now appear on the Selected Genomes menu. You can also select any of your Notebooks and include all the genomes contained in it. 5. Enter your query sequence in FASTA format. If desired, you can change the BLAST Parameters before starting the analysis. 6. Once all information is included click on Run CoGe BLAST (Figure 11). 7. The analysis output will include:
You can find links to the FASTA sequences used in this analysis in the "Sample data" section at the end of this page. |
Sequences with significant similarity to PVX_113230 were found in all the evaluated P. vivax strains, including PO1. However, the number of high-scoring segment pairs for each P. vivax genome was variable. The highest number of sequence homologs was observed in the strains: Mauritania, PO1, and Salvador-1. Sequence divergence of vir members within P. vivax seems to affect the number of high-scoring segment pairs per strain. Thus, the variation in the number of HSPs across strains further supports observations about the high sequence variation among vir homologs.
The location of HSPs appears to be slightly variable across genomes. However, we cannot confirm this patterns until the Mauritania, North Korea, Brazil I, and India VII genomes are fully assembled. Between the two fully assembled P. vivax genomes (Salvador-1 and PO1), BLAST hits were located largely in the same chromosome regions (Figure 12). As expected, a higher number of BLAST hits and a more variable genome location were observed when a less conserved vir family member (PVX_096004.1) was used as a query (analysis can be run following this link: https://genomevolution.org/r/mkcg).
Identifying microsyntenic regions (GEvo)

Changes in local genome organization can be used to ascertain the evolutionary history of a region (microsynteny). In Plasmodium, many genes related to parasite-host interactions are rapidly evolving and undergo frequent rearrangements, gain/loss events, and horizontal transfer. These evolutionary processes leave "genomic signals" by altering the local genome organization. Erythrocyte invasion is a multi-step process that represents one of the most crucial steps in the Plasmodium life cycle [32]. Recently, two P. falciparum genes (the reticulocyte-binding-like homologous protein 5 (Rh5) and the cysteine-rich protective antigen (CyRPA)) were shown to be the result of a horizontal gene transfer between P. faciparum and P. adleri progenitors within the . Remarkably, comparative genomics demonstrated that this horizontal gene transfer was localized to an 8kb segment on chromosome 4. The localized nature of this event, plus interspecific hybridization barriers suggest that the gene transfer occurred by the capture of a small segment of P. adleri progenitor genomic DNA by the P. falciparum progenitor within the Laveranian subgenus. As Rh5 and CyRPA are crucial for host erythrocyte invasion by P. falciparum, it has been proposed that the capture of these two genes conferred a strong fitness advantage that allowed the P. falciparum progenitor to infect humans [33]. In sum, the genomic region surrounding these two genes represents an excellent case study on how to examine microsyteny with CoGe.
Here, we will use CoGe’s tool GEvo to evaluate genomic properties within this region and assess the hypothesized horizontal transfer event.

| The following steps show how to use GEvo to analyze microsyntenic regions:
2. Click on GEvo or follow this link: https://genomevolution.org/coge/GEvo.pl 3. Specify a sequence for each box found under Sequence (you can specify a maximum of 25 sequences). Each box contains:
You can either import sequences for GEvo analysis by entering their gene IDs in the Name box, or you can select gene pairs for analysis directly from SynMap. 4. Click on Run GEvo. 5. The GEvo analysis will display the syntenic region between the compared genomes. 6. You can modify the parameters of the GEvo analysis in the Algorithm tab. Also, you can modify the information of the graphical display by altering the options on the Results Visualization Options tab.
|
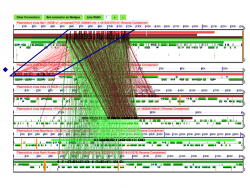
We performed a microsynteny analysis of the genome region containing Rh5 and CyRPA. The analysis was conducted using the five fully sequenced Laveranian genomes currently available: P. falciparum strains 3D7 and IT, P. reichenowi strains CDC and SY57, and P. gaboni strain SY75. Our results show that microsynteny is largely maintained in the regions surrounding Rh5 and CyRPA. We modified the Results Visualization Options tab to display background and wobble GC content for genes in this region. Neither background GC content across the region, nor wobble GC content for either Rh5 or CyRPA vary significantly (Figure 13). It has been proposed that significant changes in background or wobble GC content could be used as evidence of a horizontal transfer event. However, we did not observe such a pattern in our analyses. It is possible that a horizontal transfer event between ancestral Laveranian genomes might not be detected using this method due to the similar nucleotide composition of species in the subgenus. Therefore, an additional test might be required to further support the proposed horizontal transfer event.
We also used GEvo to further analyze regions where putative inversion breakpoints are located. Comparative analyses between P. vivax (Salvador-1) and P. vivax (PO1), and between P. vivax (Salvador-1) and P. cynomolgi show two inversion events. These events are not observed in comparisons between P. cynomolgi and P. vivax (PO1). A detailed study of the inversion breakpoints using GEvo shows genome segments of low sequence quality on P. vivax (Salvador-1) (Figure 14). This suggests that the reported inversion event might be the product of a sequencing artifact instead of a real rearrangement.
Performing synteny analyses between two genomes (SynMap)
Over evolutionary time, neighboring genes often maintain their relative position and order within a chromosomal segment. Chromosomal regions from different species that contain colinear homologs are said to be syntenic, i.e., genomic regions of shared ancestry. Changes in colinearity within syntenic regions are used to ascertain the nature, location, and extension of rearrangement events between related species. The main use of CoGE’s tool, SynMap, is to find syntenic regions where gene order is preserved. SynMap’s graphical output allows for easy and fast interpretation of these results.
| The following steps show how to analyze syntenic gene pairs with SynMap:
2. Click on Organism View or follow this link: https://genomevolution.org/coge/OrganismView.pl 3. Type a scientific name in the Search box and select the appropriate genome. Then, click on the GenomeInfo link under the Genome Information section. 4. Find the link to the SynMap tool under the Analyze section. 5. By default, SynMap will perform a self-comparison of any selected genome. This is of use when characterizing a genome or when attempting to identify the relative age of putative duplication events [34]. To analyze two different genomes, type a scientific name on the Search box of either Organism 1 or Organism 2. Once finished, click on Generate SynMap to run the analysis (Figure 15). 6. SynMap will output a graphical depiction of the syntenic regions between two genomes. There are currently two version of SynMap:
7. You can further analyze regions or genes of interest using the GEvo tool linked to SynMap. To do this, double click on a syntenic gene pair (SynMap Legacy), or select a syntenic gene pair and click on Compare in GEvo >>> (SynMap2).
https://genomevolution.org/r/lj12 (P. vivax vs. P. cynomolgi) https://genomevolution.org/r/lj1x (P. knowlesi vs. P. cynomolgi) https://genomevolution.org/r/lj1t (P. knowlesi vs. P vivax)
https://genomevolution.org/r/lq5x (P. knowlesi vs. P. malariae) https://genomevolution.org/r/lj2b (P. coatneyi vs. P. knowlesi) https://genomevolution.org/r/lq5y (P. coatneyi vs. P. malariae) https://genomevolution.org/r/lq5t (P. ovale vs. P. malariae) https://genomevolution.org/r/lq65 (P. coatneyi vs. P. ovale) https://genomevolution.org/r/lq5v (P. ovale vs. P. knowlesi) |
Identifying syntenic gene pairs
Gene position can be critical in gene expression. In many eukaryotes, expression of neighboring genes is coordinated by adjacent regulatory elements [35][36][37]. Thus, changes in gene position and order can potentially alter gene expression inside the genomic neighborhood. In P. falciparum, there is evidence that coordinated expression is absent in the highly dynamic subtelomeric regions. Furthermore, subtelomeric neighboring genes are known to form small independently expressed groups in a process thought to increase parasite’s adaptive potential [38]. It is still unknown if these transcriptional "islands" are found outside the subtelomeric regions, or even in other Plasmodium parasites. The first step to address this issue is to use tools that allow the rapid identification of changes in gene order and position. We can use SynMap to determine the origin, establish a gene’s relative location, and identify changes in gene position and order. This information can be used to later establish if patterns of coordinated expression, or lack of thereof, are prevalent across the Plasmodium genus.
Identifying chromosomal inversions, fusions, fissions and other events between two genomes
Numerous genome rearrangements have taken place throughout the evolution of the genus Plasmodium. There is a strong correlation between synteny and divergence times. In other words, the more closely related two species are, the more likely synteny will be observed between their genomes [39]. We can use SynMap to infer the putative evolutionary origin and relative location of rearrangement events across the length of the genome.
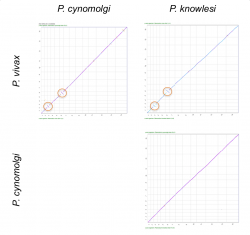
We used SynMap to confirm the relative genome location and origin of reported inversions between P. vivax, P. cynomolgi and P. knowlesi’s 3rd and 6th chromosomes. We performed pairwise comparisons to evaluate changes in genome organization amongst the three species (Figure 16). We only detected inversion events in pairwise comparisons with P. vivax (Figure 16, orange circles). This suggests that the inversion events reported on chromosomes 3 and 6 occurred after the split of P. cynomolgi and P. vivax (approximately 3.43-3.87 Mya) [40]. However, a detailed analysis of the breakpoint regions in P. vivax using GEvo (Figure 14) shows a genome segment of low sequence quality. Thus, it is possible that the inversion event reported on P. vivax could actually be an artifact.
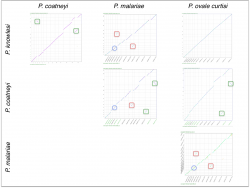
We also used SynMap to infer changes in gene order and composition amongst another group of closely related Plasmodium species. Pairwise comparisons were performed between four closely related Plasmodium parasites from the simian clade: P. ovale curtisi, P. malariae, P. coatneyi and P. knowlesi. We identified independent sets of chromosome fusion/fission events across these species. A set of fusions/fissions was found on P. malariae’s 5th and 9th chromosomes (Figure 17, red squares); another set of fusion/fission events was found on P. coatneyi’s 13th and 14th chromosomes (Figure 17, green squares). In addition, we found an inversion event located in the central region of P. malariae’s 4th chromosome (Figure 17, blue circle).
Measuring Kn/Ks values between genomes (SynMap - CodeML analysis tool)
Differences in nucleotide loci will accumulate between two genomes as the result of evolution. Nucleotide changes that alter the coded amino acid are called non-synonymous and those that do not are called synonymous. Synonymous substitutions are largely neutral and mostly reflect background evolutionary changes. On the other hand, non-synonymous substitutions are largely affected by natural selection. Under neutrality, the rate of synonymous (Ks) and non-synonymous (Kn) substitutions will be equivalent. Deviations from this expectation indicate a significant role of natural selection. Insights into trends of natural selection are gained from evaluating the Kn/Ks ratio. We observe Kn/Ks = 1 under neutrality; we observe Kn/Ks > 1 when non-synonymous substitutions are fixated at a faster rate than synonymous ones (positive selection); and, we observe Kn/Ks < 1 when new amino acid changes are eliminated (purifying selection).
The CoGe platform has the capability of calculating the Kn/Ks ratio on syntenic gene pairs across the length of a genome. CoGe’s Kn/Ks analyses can be used to:
- Identify hotspots of strong positive or purifying selection across the length of the genome.
- Establish associations between genome position (e.g. telomeres vs. centromeres) and trends of natural selection.
- Describe species- or genus-specific adaptive trends.
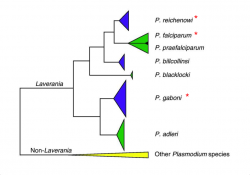
CoGe uses the CodeML analysis tool to measure the Kn/Ks ratio between two annotated genomes. The CodeML analysis tool can be accessed from SynMap. Here, we evaluated the selective trends of three closely related species from the Laveranian subgenus (Figure 18).


| The following steps show how to perform Kn/Ks analyses using SynMap’s CodeML tool:
2. Run SynMap or select a previous SynMap analysis from My Data (CoGe stores all ran analyses under a users' account). 3. Find the CodeML tool under the Analysis Options tab. Click on Calculate syntenic CDS pairs and color dots: substitution rates(s) and select Synonymous (Ks) from the dropdown menu. Repeat the analysis selecting the Non-synonymous (Kn) and (Kn/Ks) options. You can alter the display selecting a different Color Scheme, specifying Min Val. or Max Val. axis values, or changing the Log10 Transform. data option. 4. The analysis will modify the Syntenic_dotplot display to represent the distribution of the Ks, Kn or Kn/Ks values across syntenic gene pairs. A Histogram of Ks values (or Kn or Ks/Kn) will also be generated. In SynMap2, specific regions can be dynamically selected to view the Ks, Kn or Kn/Ks values.
https://genomevolution.org/r/ljhj (P. reichenowi vs. P. falciparum) https://genomevolution.org/r/ljhl (P. falciparum vs. P. gaboni) https://genomevolution.org/r/ljhq (P. reichenowi vs. P. gaboni)
https://genomevolution.org/r/lsyy (P. reichenowi vs. P. gaboni) https://genomevolution.org/r/lsz2 (P. reichenowi vs. P. falciparum) https://genomevolution.org/r/lsz5 (P. falciparum vs. P. gaboni) |
P. reichenowi and P. falciparum are thought to have diverged approximately 5.28-5.93 Mya [42]. The divergence time of either species with P. gaboni is estimated to be larger [43]. Based on these evolutionary relationships, it is expected that the number of accumulated differences in nucleotide loci will be smaller between P. reichenowi and P. falciparum, than between both species and P. gaboni.
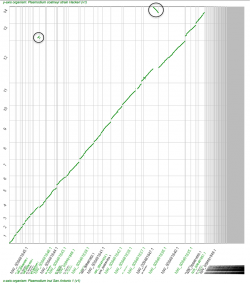
We found smaller Ks values between P. gaboni (SY57) - P. reichenowi (CDC) than between P. gaboni (SY57) - P. falciparum (3D7) (Figure 19). Also, smaller Ks values were observed between P. reichenowi - P. falciparum than between P. falciparum - P. gaboni. The same trends were observed when a different P. reichenowi strain (SY75) was used (results can be replicated in the following links: https://genomevolution.org/r/mr5u for P. reichenowi vs. P. gaboni, and https://genomevolution.org/r/lzrr for P. reichenowi vs. P. falciparum). The differences in Ks rates suggest that a recent number of synonymous substitutions occurred on the P. reichenowi genome. Genome composition and codon usage are largely similar amongst Laveranian species (Figures 10 and 24). Thus, this variation could indicate an increased mutation rate on P. reichenowi, resulting in a rapidly evolving genome compared to other Laveranian. However, the reasons for this putative increment remain unexplored.
Non-synonymous (Kn) substitution rates were largely similar between P. gaboni - P. falciparum and P. gaboni - P. reichenowi (Figure 20). Smaller Kn substitution values were observed between P. falciparum - P. reichenowi. Similar trends were seen when P. reichenowi (SY75) was used (results can be replicated in the following links: https://genomevolution.org/r/mr5z for P. reichenowi vs. P. gaboni, and https://genomevolution.org/r/mr5x for P. reichenowi vs. P. falciparum). These results suggest that a comparable rate of Kn changes occurred since the divergence of the P. reichenowi/P. falciparum ancestor. These changes were followed by a significant number of species-specific substitutions on both P. falciparum and P. reichenowi. Previous studies have found large Kn values in P. reichenowi - P. falciparum comparisons; particularly, in genes expressed during blood parasite's stages [44]. Thus, our results likely reflect Kn changes related to parasite-host interactions and adaptations to infection of different host types.
Identifying sets of syntenic genes amongst several genomes (SynFind)
Small-scale genomic rearrangements are often linked to species-specific gene gain/loss events. Family-linked rearrangements are observed amongst closely related Plasmodium species, and in occasion, at an intra-specific level. CoGe’s tool, SynFind, is used to identify gene homologs across any number of genomes, and thus can be of use to identify these rearrangements.
The evolutionary trajectory of multigene families can be difficult to infer, especially in those with a scattered organization or rapid gene turnover. While this issue is particularly prevalent in species-specific families; genus-specific families can present intricate evolutionary patterns as well. Among these, the evolutionary history of the SERA (serine repeat antigen) family is highly dynamic. This family has experienced a significant number of inter-specific contractions, expansions, and rearrangements. These patterns remain to be evaluated at an intra-specific level. We will use SynFind to study family's organization of SERA paralogs in 6 P. vivax strains.
SERA paralogs are expressed during various stages of the Plasmodium life cycle. All SERA family members code proteins with a papain-like cysteine protease motif [45]. These motifs are commonly found both inside and outside the genus Plasmodium [46][47]. One member (SERA-5), expressed during late trophozoite and schizont stages, has been considered as a promising malaria vaccine target [48]. We will use this gene sequence as a query for the SynFind analysis.
| The following steps show how to use SynFind:
2. Click on SynFind or follow this link: https://genomevolution.org/CoGe/SynFind.pl. 3. Type a scientific name of your search bar under Select Target Genomes. Organisms and genomes with names matching the search term will be displayed on the Matching Organisms menu. 4. Select the genomes of interest using Crtl+click or Command+click, then click on + Add. The genomes will appear on the Selected Genomes menu. You can also import genomes from your Notebooks. 5. Type the Name, Annotation, or Organisms on the Specify Features section. It is recommended to include as many specific terms as possible. Once done click on Search. 6. All matches to the search term and the genome where they have been found will appear in a new menu within the same section. Select all relevant Matches and the reference Genome. 7. Click on Run SynFind to start the analysis. 8. SynFind will output all syntenic regions from the reference genome and their Syntenic depth. This output can be used as a query for other CoGe tools.
GEvo results can be replicated here: https://genomevolution.org/r/mpdf |
We used Synfind to identify genes homologous to SERA-5 across 6 P. vivax genomes (Figure 21). Synfind’s output was used as a query for a GEvo analysis of the region. Our results show a conserved number of SERA paralogs in all P. vivax strains. The organization of the SERA family was different on the Brazil I strain respect to other P. vivax strains (Figure 22). Previous studies on SERA have suggested that some family members are unique to P. vivax and closely related species [49]. Our results indicate that family organization is not completely conserved on the intra-specific level. This is most evident on recently duplicated paralogs.
SynFind also identified matching segments outside the SERA multigene family. These segments belonged to hypothetical protein coding genes, ATP proteases, and uncharacterized transcripts. Papain-like cysteine protease motifs are commonly found outside both Plasmodium and the SERA family. Thus, is likely that these segments share a papain-like cysteine protease motif but are not evolutionarily related to SERA.
Identifying codon and amino acid substitution frequencies (CodeOn)

Codon and amino acid usage are significantly shaped by two factors: selection for translational efficiency and genome composition. The significance of translational selection on genome evolution varies across the genus Plasmodium. It is believed that usage of less energetically expensive amino acids provides an evolutionary advantage by decreasing energetic costs during infection [50]. On P. falciparum many highly expressed genes are majorly composed of C-ended codons despite the AT-rich genome. On the GC-rich P. vivax genome, translational selection and codon usage bias are not strongly related [51]. Genome composition is also a powerful force in protein evolution.
Here, we will use CodeOn to calculated amino acid usage across a range of GC-rich to GC-poor genomes. We will measure the effects of genome composition bias on amino acid usage across 7 Plasmodium genomes from two major clades (Laveranian and simian).

| The following steps indicate how to built amino acid usage tables using CodeOn:
2. Find the genome of interest in OrganismView or follow this link https://genomevolution.org/coge/OrganismView.pl 3. Click on CodeOn to start the analysis. After a couple of minutes, the output will show in a different tab.
https://genomevolution.org/coge/CodeOn.pl?oid=27002 (P. vivax) https://genomevolution.org/coge/CodeOn.pl?dsgid=32770 (P. cynomolgi) https://genomevolution.org/coge/CodeOn.pl?oid=26997 (P. knowlesi) https://genomevolution.org/coge/CodeOn.pl?oid=40698 (P. coatneyi)
https://genomevolution.org/coge/CodeOn.pl?oid=26992 (P. falciparum) https://genomevolution.org/coge/CodeOn.pl?oid=40801 (P. reichenowi) https://genomevolution.org/coge/CodeOn.pl?oid=40696 (P. gaboni) |
Amino acid usage trends were markedly different in species from different clades (Figure 23 and Figure 24). On the other hand, closely related Plasmodium species showed similar amino acid usage patterns.
P. vivax (Salvador-1) had the highest number of CDS with 45-55% GC content. Closely related species (P. cynomolgi, P. knowlesi, and P.coatneyi) had a higher number of CDS in the 40-45% GC tier (Figure 23). Genome composition is similar between P. cynomolgi, P. knowlesi, and P. coatneyi (Figure 9 and Figure 10). However, patterns of amino acid usage were markedly different on P. coatneyi respect to other simian species (Figure 23).
In the Laveranian subgenus, the number of CDS with 20-30% GC content was significantly larger. Amino acid usage was similar in P. falciparum (3D7) and P. reichenowi (SY57), but slightly different on P. gaboni (Figure 24). This variation is noteworthy given that the three species share a similar compositional bias (Figure 9 and Figure 10). This result suggests that compositional genome bias is a significant factor in amino acid usage on both the simian clade and Laveranian subgenus. However, we cannot discard the significance of additional factors not evaluated here.
Using Syntenic Path Assembly (SPA) to make analysis of poor or early genome assemblies easier (SynMap - SPA tool)
There is a large number of Plasmodium genomes that remain to be fully sequenced, assembled and annotated. Incomplete genomic data comes from a variety of sources:
- Genomic information published on early assembly stages.
- Partially sequenced genomes.
- Low-quality genome segments.
Sequencing projects can be slightly simplified by the use of a reference genome as a guideline for genome assembly. While unassembled and non-annotated genomes can be used in smaller-scale studies (e.g. orthologs can be identified with BLAST), there are limitations in their usability in large-scale comparative genomics.
Tools that generate preliminary assemblies have great significance in comparative analyses, especially when large amounts of genomic data become available. CoGe’s tool, Syntenic_path_assembly (SPA), creates a graphical display of syntenic gene pairs based on a reference genome. We will use SPA to assemble the P. inui genome (on scaffold level as in 2016) using the fully assembled P. coatneyi genome as a reference.
| The following steps show how to use SynMap - SPA tool:
2. Run SynMap between an assembled and a non-assembled genome (this might take longer than analyses using two fully assembled genomes). 3. After running SynMap click on the Display Options tab and find the SPA tool (Figure 25). Select the tool by clicking on the check mark next to: The Syntenic Path Assembly (SPA)? 4. After a few minutes, the incomplete genome will be assembled using the second genome as a reference.
|
SPA is extremely useful to generate quick and dirty genome assemblies; however, there are some limitations regarding assembly interpretation. We highlight two scenarios seen on the P. inui’s SPA using P. coatneyi’s genome as a reference (Figure 26).
Rearrangement events such as inversions or duplications cannot be identified using SPA. For one, several contigs can be syntenic to the same region of the reference genome without signaling a duplication event. Also, contigs syntenic to a reverse DNA strand might not reflect chromosome inversions (black circles, Figure 26).
In addition, contigs will be arranged to increase synteny between the unassembled and the reference genome. Thus, using different reference genomes will result in different preliminary assemblies. In the case of P. inui, using P. coatneyi (a closely related species) or P. falciparum (a distant species) as reference genomes will result on different assemblies. Therefore, before running SPA, the reference genomes should be selected after consideration of the biological and evolutionary relation between species. Also, interpretation of SPA assemblies might be problematic when working with transposon-rich genomes.
Overall conclusions
The number of available Plasmodium genomes has increased considerably during recent years. The increment of genomic information creates an unprecedented opportunity to study the unique genomic qualities of this genus.
Thanks to worldwide efforts, there has been a significant reduction in the number of malaria cases and malaria-related deaths between 2000 and 2015. By 2015, it was estimated that the number of malaria cases decreased from 262 million to 214 millions, and the number of malaria-related deaths from 839,000 to 438,000 [52]. There have been tremendous achievements in malaria treatment and control strategies. However, there are still numerous aspects that need further addressing in malaria research.
The intricacies of parasite-host relations in Plasmodium infection might be more complex than previously considered [53]. Humans have been infected by Plasmodium species classically considered specific of non-human primates (e.g. a single infection with P. cynomolgi [54] and various infections with P. knowlesi [55]). African primates have been infected by unique P. falciparum strains (a parasite classically considered exclusive to humans) and are proposed to act as reservoirs for this parasite [56][57]. In bird Plasmodium, the putative evolutionary time of parasite-host associations has a significant role in the development of pathogenicity and in host mortality [58]. Finally, multiple host-switch events between largely divergent host types are thought to have occurred on bat Haemosporidia [59]. These cases highlight the complexity of the Plasmodium infection landscape. Insights into the unique patterns of Plasmodium biology, epidemiology, ecology, and genetics can be obtained from molecular and comparative genomic studies.
The rapid growth of genomic information makes implementing tools that facilitate assessing genome evolutionary trends an imperative task. The services and tools provided by the CoGe platform are of considerable use in advancing Plasmodium comparative genomics. Here, we showed how various CoGe tools could be used to assess evolutionary patterns unique to Plasmodium. We also showed how to use this platform to further characterize sequenced Plasmodium genomes. Overall, we have demonstrated that CoGe’s tools can be used to address evolutionary questions such as:
- The evolutionary origins of Laveranian AT-rich genomes.
- The location and nature of genome rearrangements between Plasmodium.
- The evolutionary patterns of genes crucial in cell invasion.
- The evolutionary trends of multigene families.
Useful links
Plasmodium Notebooks in CoGe
- Link to Notebook for published Plasmodium genome data: https://genomevolution.org/coge/NotebookView.pl?lid=1753
- Link to Notebook for published P. falciparum strains: https://genomevolution.org/coge/NotebookView.pl?lid=1758
- Link to Notebook for published P. vivax strains: https://genomevolution.org/coge/NotebookView.pl?lid=1760
- Link to Notebook for published Plasmodium apicoplast data: https://genomevolution.org/coge/NotebookView.pl?lid=1754
- Link to Notebook for published Plasmodium mitochondrion data: https://genomevolution.org/coge/NotebookView.pl?lid=1756
Sample data
- Gene sequences used on CoGeBLAST analysis (obtained from PlasmoDB):
- PVX_113230.1 | Plasmodium vivax Sal-1 | variable surface protein Vir14-related (http://plasmodb.org/plasmo/app/record/gene/PVX_113230)
- PVX_096004.1 | Plasmodium vivax Sal-1 | VIR protein (http://plasmodb.org/plasmo/app/record/gene/PVX_096004)
- Gene sequence used on SynFind to inform GEvo analysis (obtained from PlasmoDB):
- PVX_003830.1 | Plasmodium vivax Sal-1 | serine-repeat antigen 5 (SERA) (http://plasmodb.org/plasmo/app/record/gene/PVX_003830)
- Gene sequences used on CoGeBLAST to inform GEvo analysis (obtained from PlasmoDB):
- PF3D7_0424100.1 | Plasmodium falciparum 3D7 | reticulocyte binding protein homologue 5 (http://plasmodb.org/plasmo/app/record/gene/PF3D7_0424100)
- PVX_096410.1 | Plasmodium vivax Sal-1 | cysteine repeat modular protein 2, putative (http://plasmodb.org/plasmo/app/record/gene/PVX_096410)
References
- ↑ Jackson AP. 2015. Preface. The evolution of parasite genomes and the origins of parasitism. Parasitology. 142 Suppl 1:S1-5. https://www.ncbi.nlm.nih.gov/pubmed/25656359
- ↑ Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW, Burkot TR, Harbach RE, Hay SI. 2012. A global map of dominant malaria vectors. Parasit Vectors. 5:69. https://www.ncbi.nlm.nih.gov/pubmed/22475528
- ↑ DeBarry JD, Kissinger JC. 2011. Jumbled Genomes: Missing Apicomplexan Synteny. Mol Biol Evol. 2011 Oct; 28(10): 2855–2871. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3176833/
- ↑ Carlton JM, Perkins SL, Deitsch KW. 2013. Malaria Parasites. Caister Academic Press
- ↑ Prugnolle F, Durand P, Ollomo B, Duval L, Ariey F, Arnathau C, Gonzalez JP, Leroy E, Renaud F. 2011. A Fresh Look at the Origin of Plasmodium falciparum, the Most Malignant Malaria Agent. PLoS Pathog. 7: e1001283. http://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1001283
- ↑ Prugnolle F, Rougeron V, Becquart P, Berry A, Makanga B, Rahola N, Arnathau C, Ngoubangoye B, Menard S, Willaume E, Ayala FJ, Fontenille D, Ollomo B, Durand P, Paupy C, Renaud F. 2013. Diversity, host switching and evolution of Plasmodium vivax infecting African great apes. Proc Natl Acad Sci U S A. 110:8123-8. https://www.ncbi.nlm.nih.gov/pubmed/23637341
- ↑ Buscaglia CA, Kissinger JC, Agüero F. 2015. Neglected Tropical Diseases in the Post-Genomic Era. Trends Genet. 31:539-55. https://www.ncbi.nlm.nih.gov/pubmed/26450337
- ↑ Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW. 2016. GenBank. Nucleic Acids Res. 44: D67–D72. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4702903/
- ↑ Aurrecoechea C, Brestelli J, Brunk BP, Dommer J, Fischer S, Gajria B, Gao X, Gingle A, Grant G, Harb OS, Heiges M, Innamorato F, Iodice J, Kissinger JC, Kraemer E, Li W, Miller JA, Nayak V, Pennington C, Pinney DF, Roos DS, Ross C, Stoeckert CJ Jr, Treatman C, Wang H. 2009. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 37:D539-43. https://www.ncbi.nlm.nih.gov/pubmed/18957442
- ↑ Logan-Klumpler FJ, De Silva N, Boehme U, Rogers MB, Velarde G, McQuillan JA, Carver T, Aslett M, Olsen C, Subramanian S, Phan I, Farris C, Mitra S, Ramasamy G, Wang H, Tivey A, Jackson A, Houston R, Parkhill J, Holden M, Harb OS, Brunk BP, Myler PJ, Roos D, Carrington M, Smith DF, Hertz-Fowler C, Berriman M. 2012. GeneDB--an annotation database for pathogens. Nucleic Acids Res. 40:D98-108. https://www.ncbi.nlm.nih.gov/pubmed/22116062
- ↑ Bensch S, Hellgren O, Pérez-Tris J. 2009. MalAvi: a public database of malaria parasites and related haemosporidian in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour. 9:1353-8. https://www.ncbi.nlm.nih.gov/pubmed/21564906
- ↑ Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 419:498-511
- ↑ Wu H, Zhang Z, Hu S, Yu S. 2012. On the molecular mechanism of GC content variation among eubacterial genomes. Biol Direct. 2012; 7: 2. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3274465/
- ↑ Lassalle F, Périan S, Bataillon T, Nesme X, Duret L, Daubin V. 2015. GC-Content Evolution in Bacterial Genomes: The Biased Gene Conversion Hypothesis Expands. PLoS Genet. 11: e1004941. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4450053/
- ↑ Šmarda P, Bureš P, Horová L, Leitch IJ, Mucina L, Pacini E, Tichý L, Grulich V, Rotreklováa O. 2014. Ecological and evolutionary significance of genomic GC content diversity in monocots. Proc Natl Acad Sci U S A. 111: E4096–E4102. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4191780/
- ↑ Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 419:498-511
- ↑ Jackson AP. 2015. Preface. The evolution of parasite genomes and the origins of parasitism. Parasitology. 142 Suppl 1:S1-5. https://www.ncbi.nlm.nih.gov/pubmed/25656359
- ↑ DeBarry JD, Kissinger JC. 2011. Jumbled Genomes: Missing Apicomplexan Synteny. Mol Biol Evol. 2011 Oct; 28(10): 2855–2871. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3176833/
- ↑ Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 419:498-511
- ↑ Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, Caler E, Crabtree J, Angiuoli SV, Merino EF, Amedeo P, Cheng Q, Coulson RM, Crabb BS, Del Portillo HA, Essien K, Feldblyum TV, Fernandez-Becerra C, Gilson PR, Gueye AH, Guo X, Kang'a S, Kooij TW, Korsinczky M, Meyer EV, Nene V, Paulsen I, White O, Ralph SA, Ren Q, Sargeant TJ, Salzberg SL, Stoeckert CJ, Sullivan SA, Yamamoto MM, Hoffman SL, Wortman JR, Gardner MJ, Galinski MR, Barnwell JW, Fraser-Liggett CM. 2008. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 455:757-63. https://www.ncbi.nlm.nih.gov/pubmed/18843361
- ↑ Nikbakht H, Xia X, Hickey DA. 2014. The evolution of genomic GC content undergoes a rapid reversal within the genus Plasmodium. Genome. 9:507-511. https://www.ncbi.nlm.nih.gov/pubmed/25633864
- ↑ Hayakawa T, Culleton R, Otani H, Horii T, Tanabe K. 2008. Big bang in the evolution of extant malaria parasites. Mol Biol Evol. 10:2233-9. https://www.ncbi.nlm.nih.gov/pubmed/18687771
- ↑ Bensch S, Canbäck B, DeBarry JD, Johansson T, Hellgren O, Kissinger JC, Palinauskas V, Videvall E, Valkiūnas G. 2016. The Genome of Haemoproteus tartakovskyi and Its Relationship to Human Malaria Parasites. Genome Biol Evol. 8:1361-73.https://www.ncbi.nlm.nih.gov/pubmed/27190205
- ↑ Singh V, Gupta P, Pande V. 2014. Revisiting the multigene families: Plasmodium var and vir genes. J Vector Borne Dis. 51:75-81. https://www.ncbi.nlm.nih.gov/pubmed/24947212
- ↑ Niang M, Yan Yam X, Preiser PR. 2009. The Plasmodium falciparum STEVOR multigene family mediates antigenic variation of the infected erythrocyte. PLoS Pathog. 5:e1000307. https://www.ncbi.nlm.nih.gov/pubmed/19229319
- ↑ Witmer K, Schmid CD, Brancucci NM, Luah YH, Preiser PR, Bozdech Z, Voss TS. 2012. Analysis of subtelomeric virulence gene families in Plasmodium falciparum by comparative transcriptional profiling. Mol Microbiol. 84:243-59. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3491689/
- ↑ Petter M, Bonow I, Klinkert MQ. 2008. Diverse expression patterns of subgroups of the rif multigene family during Plasmodium falciparum gametocytogenesis. PLoS One. 3:e3779. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0003779
- ↑ Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, Caler E, Crabtree J, Angiuoli SV, Merino EF, Amedeo P, Cheng Q, Coulson RM, Crabb BS, Del Portillo HA, Essien K, Feldblyum TV, Fernandez-Becerra C, Gilson PR, Gueye AH, Guo X, Kang'a S, Kooij TW, Korsinczky M, Meyer EV, Nene V, Paulsen I, White O, Ralph SA, Ren Q, Sargeant TJ, Salzberg SL, Stoeckert CJ, Sullivan SA, Yamamoto MM, Hoffman SL, Wortman JR, Gardner MJ, Galinski MR, Barnwell JW, Fraser-Liggett CM. 2008. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 455:757-63. https://www.ncbi.nlm.nih.gov/pubmed/18843361
- ↑ Lopez FJ, Bernabeu M, Fernandez-Becerra C, del Portillo HA. 2013. A new computational approach redefines the subtelomeric vir superfamily of Plasmodium vivax. BMC Genomics. 14:8. https://www.ncbi.nlm.nih.gov/pubmed/?term=A+new+computational+approach+redefines+the+subtelomeric+vir+superfamily+of+Plasmodium+vivax
- ↑ Fernandez-Becerra C, Yamamoto MM, Vêncio RZ, Lacerda M, Rosanas-Urgell A, del Portillo HA. 2009. Plasmodium vivax and the importance of the subtelomeric multigene vir superfamily. Trends Parasitol. 2009 25:44-51. https://www.ncbi.nlm.nih.gov/pubmed/19036639
- ↑ Neafsey DE, Galinsky K, Jiang RH, Young L, Sykes SM, Saif S, Gujja S, Goldberg JM, Young S, Zeng Q, Chapman SB, Dash AP, Anvikar AR, Sutton PL, Birren BW, Escalante AA, Barnwell JW, Carlton JM. 2012. The malaria parasite Plasmodium vivax exhibits greater genetic diversity than Plasmodium falciparum. Nat Genet. 44:1046-50. https://www.ncbi.nlm.nih.gov/pubmed/22863733
- ↑ Cowman AF, Crabb BS. 2006. Invasion of red blood cells by malaria parasites. Cell. 124:755-66. https://www.ncbi.nlm.nih.gov/pubmed/16497586
- ↑ Sundararaman SA, Plenderleith LJ, Liu W, Loy DE, Learn GH, Li Y, Shaw KS, Ayouba A, Peeters M, Speede S, Shaw GM, Bushman FD, Brisson D, Rayner JC, Sharp PM, Hahn BH. 2016. Genomes of cryptic chimpanzee Plasmodium species reveal key evolutionary events leading to human malaria. Nat Commun. 7:11078. https://www.ncbi.nlm.nih.gov/pubmed/27002652
- ↑ Tang H, Lyons E. 2012. Unleashing the Genome of Brassica Rapa. Front Plant Sci. 3: 172. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3408644/
- ↑ Ghanbarian AT, Hurst LD. 2015. Neighboring Genes Show Correlated Evolution in Gene Expression. Mol Biol Evol. doi:10.1093/molbev/msv053http://mbe.oxfordjournals.org/content/early/2015/04/01/molbev.msv053.full
- ↑ De S, Teichmann SA, Babu MM. 2009. The impact of genomic neighborhood on the evolution of human and chimpanzee transcriptome. Genome Res. 19(5): 785–794. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2675967/
- ↑ Michalak P. 2008. Coexpression, coregulation, and cofunctionality of neighboring genes in eukaryotic genomes. Genomics. 91:(43–248) http://www.sciencedirect.com/science/article/pii/S0888754307002807
- ↑ Rovira-Graells N, Gupta AP, Planet E, Crowley VM, Mok S, Ribas de Pouplana L, Preiser PR, Bozdech Z, Cortés A. 2012. Transcriptional variation in the malaria parasite Plasmodium falciparum. Genome Res. 5:925-38. https://www.ncbi.nlm.nih.gov/pubmed/22415456
- ↑ Tachibana SI, Sullivan SA, Kawai S, Nakamura S, Kim HR, Goto N, Arisue N, Palacpac NM, Honma H, Yagi M, Tougan T, Katakai Y, Kaneko O, Mita T, Kita K, Yasutomi Y, Sutton PL, Shakhbatyan R, Horii T, Yasunaga T, Barnwell JB, Escalante AA, Carlton JM, Tanabe K. 2012. Plasmodium cynomolgi genome sequences provide insight into Plasmodium vivax and the monkey malaria clade. Nat Genet. 44: 1051–1055. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3759362/
- ↑ Pacheco MA, Reid MJ, Schillaci MA, Lowenberger CA, Galdikas BM, Jones-Engel L, Escalante AA. 2012. The origin of malarial parasites in orangutans. PLoS One. 7:e34990. https://www.ncbi.nlm.nih.gov/pubmed/22536346
- ↑ Rayner JC, Liu W, Peeters M, Sharp PM, Hahn BH. 2011. A plethora of Plasmodium species in wild apes: a source of human infection? Trends Parasitol. 27:222-9. https://www.ncbi.nlm.nih.gov/pubmed/21354860?dopt=Abstract&holding=npg
- ↑ Pacheco MA, Reid MJ, Schillaci MA, Lowenberger CA, Galdikas BM, Jones-Engel L, Escalante AA. 2012. The origin of malarial parasites in orangutans. PLoS One. 7:e34990. https://www.ncbi.nlm.nih.gov/pubmed/22536346
- ↑ Sundararaman SA, Plenderleith LJ, Liu W, Loy DE, Learn GH, Li Y, Shaw KS, Ayouba A, Peeters M, Speede S5, Shaw GM, Bushman FD, Brisson D, Rayner JC, Sharp PM, Hahn BH. 2016. Genomes of cryptic chimpanzee Plasmodium species reveal key evolutionary events leading to human malaria. Nat Commun. 7:11078. https://www.ncbi.nlm.nih.gov/pubmed/27002652
- ↑ Otto TD, Rayner JC, Böhme U, Pain A, Spottiswoode N, Sanders M, Quail M, Ollomo B, Renaud F, Thomas AW, Prugnolle F, Conway DJ, Newbold C, Berriman M. 2014. Genome sequencing of chimpanzee malaria parasites reveals possible pathways of adaptation to human hosts. Nat Commun. 5:4754. https://www.ncbi.nlm.nih.gov/pubmed/25203297
- ↑ Arisue N, Kawai S, Hirai M, Palacpac NM, Jia M, Kaneko A, Tanabe K, Horii T. 2011. Clues to Evolution of the SERA Multigene Family in 18 Plasmodium Species. PLoS One. 6: e17775. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0017775
- ↑ Prasad R, Atul, Soni A, Puri SK, Sijwali PS. 2012. Expression, characterization, and cellular localization of knowpains, papain-like cysteine proteases of the Plasmodium knowlesi malaria parasite. PLoS One. 12:e51619. https://www.ncbi.nlm.nih.gov/pubmed/23251596
- ↑ Brömme D. 2001. Papain-like cysteine proteases. Curr Protoc Protein Sci. 21. doi: 10.1002/0471140864.ps2102s21. https://www.ncbi.nlm.nih.gov/pubmed/18429163
- ↑ Arisue N, Hirai M, Arai M, Matsuoka H, Horii T. 2007. Phylogeny and evolution of the SERA multigene family in the genus Plasmodium. J Mol Evol. 65:82-91. http://link.springer.com/article/10.1007%2Fs00239-006-0253-1
- ↑ Arisue N, Kawai S, Hirai M, Palacpac NM, Jia M, Kaneko A, Tanabe K, Horii T. 2011. Clues to Evolution of the SERA Multigene Family in 18 Plasmodium Species. PLoS One. 6: e17775. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0017775
- ↑ Peixoto L, Fernández V, Musto H. 2004. The effect of expression levels on codon usage in Plasmodium falciparum. Parasitology. 128:245-51. https://www.ncbi.nlm.nih.gov/pubmed/15074874
- ↑ Yadav MK, Swati D. 2012. Comparative genome analysis of six malarial parasites using codon usage bias based tools. Bioinformation. 8:1230-9. https://www.ncbi.nlm.nih.gov/pubmed/23275725
- ↑ World Health Organization. (2015). World Malaria Report 2015. Retrieved from http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/
- ↑ Garamszegi LZ. 2009. Patterns of co-speciation and host switching in primate malaria parasites. Malar J. 110. doi: 10.1186/1475-2875-8-110. https://www.ncbi.nlm.nih.gov/pubmed/19463162
- ↑ Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM. 2014. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar J. 13: 68. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3937822/
- ↑ Singh B, Daneshvar C. 2013. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev. 26:165-84. https://www.ncbi.nlm.nih.gov/pubmed/23554413
- ↑ Prugnolle F, Durand P, Neel C, Ollomo B, Ayala FJ, Arnathau C, Etienne L, Mpoudi-Ngole E, Nkoghe D, Leroy E, Delaporte E, Peeters M, Renaud F. 2010. African great apes are natural hosts of multiple related malaria species, including Plasmodium falciparum. Proc Natl Acad Sci U S A. 107:1458-63. https://www.ncbi.nlm.nih.gov/pubmed/20133889
- ↑ Duval L, Fourment M, Nerrienet E, Rousset D, Sadeuh SA, Goodman SM, Andriaholinirina NV, Randrianarivelojosia M, Paul RE, Robert V, Ayala FJ, Ariey F. 2010. African apes as reservoirs of Plasmodium falciparum and the origin and diversification of the Laverania subgenus. Proc Natl Acad Sci U S A. 107:10561-6. https://www.ncbi.nlm.nih.gov/pubmed/20498054
- ↑ Krizanauskiene A, Hellgren O, Kosarev V, Sokolov L, Bensch S, Valkiunas G. 2006. Variation in host specificity between species of avian haemosporidian parasites: evidence from parasite morphology and cytochrome B gene sequences. J Parasitol. 6:1319-24. https://www.ncbi.nlm.nih.gov/pubmed/17304814
- ↑ Duval L, Robert V, Csorba G, Hassanin A, Randrianarivelojosia M, Walston J, Nhim T, Goodman SM, Ariey F. 2007. Multiple host-switching of Haemosporidia parasites in bats. Malar J. 6:157. https://www.ncbi.nlm.nih.gov/pubmed/18045505