Difference between revisions of "Expression Analysis Pipeline"
From CoGepedia
m |
|||
| (10 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
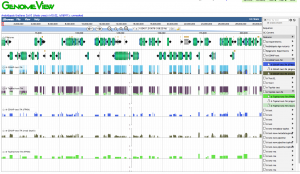
| + | [[File:Screen_Shot_2014-03-04_at_9.50.01_AM.png|thumb|300px|right|overview of various types of mapping of data from RNASeq]] [[File:Screen Shot 2014-04-07 at 2.27.54 PM.png|thumb|300px|Comparing the results of data mapped with GSNAP versus Tophat/Bowtie2]] | ||
| + | |||
CoGe can generate gene/transcript expression measurements given a FASTQ input and an annotated genome. Thanks to [http://www.skraelingmountain.com/ James Schnable], creator of [http://qteller.com/ qTeller], for help developing this pipeline! | CoGe can generate gene/transcript expression measurements given a FASTQ input and an annotated genome. Thanks to [http://www.skraelingmountain.com/ James Schnable], creator of [http://qteller.com/ qTeller], for help developing this pipeline! | ||
| + | ==Workflow== | ||
When a FASTQ file of sequence reads is loaded in [[LoadExperiment]] and associated with an annotated genome, the following analysis steps are performed: | When a FASTQ file of sequence reads is loaded in [[LoadExperiment]] and associated with an annotated genome, the following analysis steps are performed: | ||
# The FASTQ file is verified for correct format. | # The FASTQ file is verified for correct format. | ||
| − | # [http://code.google.com/p/cutadapt/ CutAdapt] is run to trim adapter sequence from the reads (parameters: -q 25 --quality | + | # [http://code.google.com/p/cutadapt/ CutAdapt] is run to trim adapter sequence from the reads (parameters: -q 25 -m 17). |
| − | # [http://research-pub.gene.com/gmap/ GMAP] is run to index the reference genome sequence. | + | ## -q: Trim low-quality ends from reads before adapter removal. The algorithm is the same as the one used by BWA (Subtract CUTOFF from all qualities; compute partial sums from all indices to the end of the sequence; cut sequence at the index at which the sum is minimal) |
| − | # [http://research-pub.gene.com/gmap/ GSNAP] is run to align the reads to the reference sequence (parameters: -n 5 --format=sam -Q --gmap-mode=none --nofails). | + | ##-m: Discard trimmed reads that are shorter than LENGTH. Reads that are too short even before adapter removal are also discarded. In colorspace, an initial primer is not counted (default: 0). |
| − | # [http://samtools.sourceforge.net/ SAMtools] is run to compute per-position read depth of the resulting alignment ( | + | # [http://research-pub.gene.com/gmap/ GMAP] or [http://bowtie-bio.sourceforge.net/bowtie2/index.shtml Bowtie2] is run to index the reference genome sequence, depending on your choice. |
| + | # [http://research-pub.gene.com/gmap/ GSNAP] or [http://tophat.cbcb.umd.edu/ TopHat] is run to align the reads to the reference sequence (GSNAP parameters: -N 1 -n 5 --format=sam -Q --gmap-mode=none --nofails, TopHat parameters: -g 1). | ||
| + | # [http://samtools.sourceforge.net/ SAMtools] is run to compute per-position read depth of the resulting alignment (depth -q 20). | ||
# [http://cufflinks.cbcb.umd.edu/ Cufflinks] is run to compte per-transcript FPKM (parameters: -p 24). | # [http://cufflinks.cbcb.umd.edu/ Cufflinks] is run to compte per-transcript FPKM (parameters: -p 24). | ||
# The per-position read depth and per-transcript FPKM values are log transformed and normalized between 0 and 1 for loading. | # The per-position read depth and per-transcript FPKM values are log transformed and normalized between 0 and 1 for loading. | ||
| Line 14: | Line 19: | ||
TBD: how to do this ... | TBD: how to do this ... | ||
| − | |||
| − | + | ==Video Tutorial== | |
{{#ev:youtube|3fNyHGB02dM}} | {{#ev:youtube|3fNyHGB02dM}} | ||
| − | + | ||
| + | ==Demo fastq data for Arabidopsis Col-0:== | ||
** 0.17M reads: http://de.iplantcollaborative.org/dl/d/2F807292-34CC-4C8E-96E3-3E668A304D23/test_rna_seq_data_0.17M_reads.fastq | ** 0.17M reads: http://de.iplantcollaborative.org/dl/d/2F807292-34CC-4C8E-96E3-3E668A304D23/test_rna_seq_data_0.17M_reads.fastq | ||
** 1M reads: http://de.iplantcollaborative.org/dl/d/EFD4F983-80B1-4388-94C4-AD78E73D2795/test_rna_seq_data_1M_reads.fastq | ** 1M reads: http://de.iplantcollaborative.org/dl/d/EFD4F983-80B1-4388-94C4-AD78E73D2795/test_rna_seq_data_1M_reads.fastq | ||
** 7.6M reads: http://de.iplantcollaborative.org/dl/d/9F6602D6-C66B-4C97-A72A-180AAE55AF95/test_rna_seq_data_7.6M_reads.fastq | ** 7.6M reads: http://de.iplantcollaborative.org/dl/d/9F6602D6-C66B-4C97-A72A-180AAE55AF95/test_rna_seq_data_7.6M_reads.fastq | ||
Latest revision as of 15:40, 5 January 2016
CoGe can generate gene/transcript expression measurements given a FASTQ input and an annotated genome. Thanks to James Schnable, creator of qTeller, for help developing this pipeline!
Workflow
When a FASTQ file of sequence reads is loaded in LoadExperiment and associated with an annotated genome, the following analysis steps are performed:
- The FASTQ file is verified for correct format.
- CutAdapt is run to trim adapter sequence from the reads (parameters: -q 25 -m 17).
- -q: Trim low-quality ends from reads before adapter removal. The algorithm is the same as the one used by BWA (Subtract CUTOFF from all qualities; compute partial sums from all indices to the end of the sequence; cut sequence at the index at which the sum is minimal)
- -m: Discard trimmed reads that are shorter than LENGTH. Reads that are too short even before adapter removal are also discarded. In colorspace, an initial primer is not counted (default: 0).
- GMAP or Bowtie2 is run to index the reference genome sequence, depending on your choice.
- GSNAP or TopHat is run to align the reads to the reference sequence (GSNAP parameters: -N 1 -n 5 --format=sam -Q --gmap-mode=none --nofails, TopHat parameters: -g 1).
- SAMtools is run to compute per-position read depth of the resulting alignment (depth -q 20).
- Cufflinks is run to compte per-transcript FPKM (parameters: -p 24).
- The per-position read depth and per-transcript FPKM values are log transformed and normalized between 0 and 1 for loading.
- The three results (raw alignment, per-position read depth, and per-transcript FPKM) are loaded as separate Experiments into a Notebook.
Genomes for which this analysis has been performed can have features imported into qTeller. TBD: how to do this ...
Video Tutorial
Demo fastq data for Arabidopsis Col-0:
- 0.17M reads: http://de.iplantcollaborative.org/dl/d/2F807292-34CC-4C8E-96E3-3E668A304D23/test_rna_seq_data_0.17M_reads.fastq
- 1M reads: http://de.iplantcollaborative.org/dl/d/EFD4F983-80B1-4388-94C4-AD78E73D2795/test_rna_seq_data_1M_reads.fastq
- 7.6M reads: http://de.iplantcollaborative.org/dl/d/9F6602D6-C66B-4C97-A72A-180AAE55AF95/test_rna_seq_data_7.6M_reads.fastq